Nosema Ceranae—Not Your Father’s Nosema!
Nosema Ceranae—Not Your Father’s Nosema!
Randy Oliver
ScientificBeekeeping.com
First published in Bee Culture
Published in ABJ in Jan. 2009
Most beekeepers are aware that a new form of nosema has established itself in North America (and throughout the world). Amazingly, Nosema ceranae was able to spread worldwide without anyone even noticing until recently—it has been in the U.S. since at least 1995, but wasn’t reported until 2007! Since that time, we have learned a bit about this new disease, but still have more questions than answers.
Nosemas are microscopic fungi that typically parasitize insects. For example Nosema locustae parasitizes grasshoppers, and is used as a biocontrol against grasshopper infestations. Various bee species also have their own nosemas—each generally adapted for a specific host. The European honey bee, which most of us keep, has long carried Nosema apis. Until recently, if beekeepers referred to “nosema,” that was the species that they meant. N. apis is normally a relatively benign parasite of honey bees. Its infection level generally spikes in fall and early spring, with the effects being most intense in areas with long, cold winters in which bees are confined in the hive without the ability to enjoy cleansing flights.
Most healthy colonies can handle a Nosema apis infection as long as they get good nutrition. However, N. apis can be a serious problem when poor weather prevents bees from foraging for pollen (or if colonies are foraging on non nutritious pollen), and cold weather stresses the older bees. It can also be a problem in queens and packages—the chilling and confinement of the bees in queen cages packages can lead to sudden spread of infection, and to premature supersedure of queens.
In order to prevent nosema problems, Northern beekeepers, and Southern queen producers, regularly feed the antifungal medication fumagillin (Fumagilin-B®, which replaced the older Fumadil-B) in gallons of heavy syrup in the fall and/or spring. This prophylactic practice, along with various husbandry practices (such as keeping colonies in sunny winter locations) have long been successful for controlling problems with N. apis.
Enter Nosema ceranae—originally a parasite of the Eastern honey bee, Apis cerana. Just as the varroa mite jumped from Apis cerana to the European honey bee, N. ceranae did the same trick. The problem is, that when a parasite jumps from one host to another (often “sister” species, as in this case), the new host does not have defenses against the new parasite (as you’ve likely noticed in the case of varroa). So a minor parasite of the original host can become a highly virulent parasite of the new host until nature (or selective breeding) establish a sustainable host-parasite relationship (or, in some devastating cases, the new host goes extinct).
The big differences between the sister species of nosema are that N. apis infection “disappears” during summer (and generally winter), and that it is restricted to infecting the cells lining the gut of older bees. N. ceranae, on the other hand, maintains infection all year long, and can move right into the bees’ body tissues.
So just how virulent is N. ceranae in the European honey bee? Initial reports from Dr. Mariano Higes’ lab in Spain were that N. ceranae was invariably devastating to colonies—a virtual “kiss of death” if left untreated. He demonstrated that the massive colony losses in Spain coincided with the appearance of N. ceranae in about 2001. He also reported that bees inoculated with N. ceranae would die within days. These findings were repoarted just as the U.S. began suffering from Colony Collapse Disorder (CCD), and the symptoms of the disorder were nearly identical to the symptoms of N. ceranae collapse as reported by the Higes team. Furthermore, when the Dr. Diana Cox-Foster’s team reported on CCD, they found N. ceranae in 100% of the CCD colonies that they tested!
Although the initial evidence looked damning, closer inspection raised questions as to whether N. ceranae was indeed the cause of the problem. Many colonies that collapsed showed no sign of N. ceranae, and many with N. ceranae appeared to be thriving. The following year Dr. Antonio Pajuelo (also in Spain) released a study that found that when natural forage was adequate, that neither fumagillin treatment for, nor the addition of protein or vitamin supplements, had any significant effect upon survival or production of colonies infected with N. ceranae!
I’ve discussed these discrepancies with Dr. Higes. He feels that colonies can handle moderate infections as long as the queen is able to sustain egglaying to offset the continued premature death of infected foragers. Collapse may not occur for months, or maybe more than a year after the colony becomes infected. A new young queen, or supersedure queen, may give the colony a “second wind,” but if the queen begins to fail, the colony will succumb. His recent studies have found that during this period of inapparent infection, colonies may exhibit a 50-70% loss of honey production.
In my own trials, I have also seen colonies that appear to thrive and produce honey (and swarms) while infected with N. ceranae. In speaking with some (but not all) large commercial beekeepers, they are not particularly concerned with moderate infection levels.
So what’s up? Is N. ceranae devastating or benign? It appears that it can be either, depending upon extenuating circumstances. The first factor that comes to mind is the influence of nutrition. Several studies have linked the virulence of N. apis to poor nutrition (although a recent study by Drs. Matilla and Otis was somewhat contrary), and research by Drs. Frank Eischen, as well as Pajuelo’s study, suggest that the same applies for N. ceranae. However, Dr. Higes feels strongly that there is no nutritional connection with N. ceranae collapse.
A second possible factor is the presence of viruses. Several of the bee viruses are closely associated with nosema infections—the viruses apparently gain entry past the bees’ immune defenses through the nosema-infected gut. This may be yet another case where one parasite (such as varroa or tracheal mites) do not kill the host directly, but either stress the host, disable its immune response, or allow transmission of a virus, so the virus(es) actually deliver the coup de grace. However, Dr. Higes was not able to associate any of the “regular” bee viruses with collapse of colonies in Spain.
Or, a third possibility is that the strains of N. ceranae and bees here in the U.S. interact differently (remember, N. ceranae has been confirmed to have been in the U.S. for at least ten years).
Now that I’ve totally confused you, what should you as a beekeeper be doing about N. ceranae? Well that’s the $64,000 question for beekeepers this year. Unfortunately, the scientific research community got caught flatfooted by N. ceranae, and don’t yet have all the answers.
I’ve been following the progress of research on this subject worldwide, and have performed a bit of research of my own (for far more information on this subject, please refer to the nosema articles on my website). Since we have little specific information on biotechnical methods of control for N. ceranae (such as colony management, comb disinfection, etc.), the beekeeper is left with four main things that he/she can do:
1. Make sure that your colonies receive good nutrition.
2. Breed from nosema-resistant bees.
3. Monitor nosema levels by periodic sampling.
4. Treat if necessary with medication.
Let’s cover these items one at a time (for convenience, we’ll get Nutrition and Breeding out of the way first, so that I can concentrate on Sampling and Treatment).
Nutrition
I’ve covered this subject in a previous article in this magazine—“nutrition” largely equates to colony protein levels, and protein levels equate with general colony health and disease resistance. Optimum nutritional input consists of a nectar flow, plus ample pollen from a variety of flowers. Some plants, such as sunflowers, dandelion, corn, and blueberries produce nutritionally-incomplete pollen, and colonies will go downhill if these plants are their sole source of pollen.
Other times that colonies suffer from low protein levels are any time that a colony full of hungry brood suffers from loss of daily pollen income. This can happen when the forage plants stop blooming, the crop is cut, or poor weather restricts foraging. It can also occur during winter or drought, during intense honeyflows, or immediately after a flow.
In these cases, feeding supplemental protein to your colonies may be of great benefit. A number of products are on the market, or you can mix your own brewers yeast based supplement.
Breeding
Long-term, we must realize that the honey bee Apis mellifera has not yet had much time to develop natural resistance to its new parasite Nosema ceranae. However, Danish breeders have demonstrated that natural resistance to N. apis can be selected for relatively quickly. There is no reason to expect that we can’t do the same for N. ceranae.
Sampling
First and foremost, there’s no sense worrying about N. ceranae if your bees are not infected. Unfortunately, there is no way to tell if they are infected without inspection of their gut contents under a microscope. This sort of inspection is frequently done by a lab, but I highly encourage beekeepers to learn to do it themselves. In order to make it easy for you, I have complete instructions on my website. You can use any microscope that can view at 400x magnification, but you generally get what you pay for. It is much easier to view spores with a good scope. If you’re going to look at a lot of samples, I recommend a quality binocular scope (two eyepieces).
Check my website for features to look for in a scope. I’ve checked a number of brands, and am impressed by the Omano OM36 scope (http://www.microscope.com/beekeeper-special-omano-om36l-package-p-339.html). If you want to do field sampling on the back of your truck bed in outyards, then get the portable model OM36L for an extra fifty bucks.
The key points in sampling are to take a consistent sample of enough bees, and to quantify the spore count per bee by using a standard dilution. The consistent sample should always be taken of bees at the entrance to the colony near midday. These points are important since you want to sample the oldest bees (the foragers), which are most infected, and because infected bees tend not to fly in the morning. Samples of bees taken from inside the hive will have much lower spore counts (by a factor of 10 to 20), and cannot be compared to entrance samples or used for treatment threshold counts.
You should sample at least 25 bees per colony—50 or more is much more accurate. The reason for this is that generally only a low percentage of bees in a colony will actually be infected—with smaller samples, a single infected bee (or the absence thereof) can greatly skew your spore count, which is the average number of spores per bee. This average is an artificial number; in reality, most bees will have zero spores, and a portion will be highly infected.
I find that the quickest and easiest method of taking and processing nosema samples is to use a modified portable vacuum to suck up a dozen or so bees from the entrances of at least half the colonies in each yard (don’t include any “crawlers” off the ground—they will skew the count). Alternatively, you can brush some off the landing boards into a cup of rubbing alcohol (so they don’t fly back out). The point is to get a large, representative sample from each colony, or in the case of commercial beekeepers, each yard. All samples of bees should be immersed in rubbing alcohol, sealed in a jar (glass or plastic), and labeled.
The Suck-a-Bee—a portable vacuum modified to collect bees directly into alcohol. Plans for this timesaving device are posted in the Nosema Section of this website.
Once home, you need to count the number of bees in the sample, so that you can make the proper dilution of 1ml per bee. Luckily, I’ve found a shortcut for counting large samples of bees that is accurate enough for treatment decisions. Simply drain the alcohol off the bees, and settle their damp bodies down into a measuring cup by tamping the bottom of the cup onto your palm. It just so happens that damp dead bees pack down to about 3 bees per milliliter, and since you want to add 1ml per bee, you can simply dilute them with 3 times the volume of water that there is volume of bees. For example, if you have a quarter cup of bees, then add three-quarters of a cup of water (for a half cup, add 1-1/2 cups of water).
A quick volumetric method of “counting” the number of drained bees in a sample. There are roughly 3 bees per milliliter. So this sample of 100ml of bees would contain about 300 bees, and need 300 ml of water to dilute them for a standardized spore count. In general, use 3x the volume of water as settled bees.
The more astute of you will also have realized that the alcohol drained from those bees will contain all the varroa mites (if you shake up the jar). So you’ve got a chance to do a “twofer”—take a nosema sample, and an accurate mite infestation level yard sample at the same time!
The bees need to be ground up to release the spores in their guts. The quickest (and most consistent) way that I’ve found is to use a kitchen blender (I’ve tried the 12v blender that we use to make Margaritas on the tailgate, but it doesn’t work as well), and chop them up at the slowest setting (you don’t want to totally liquefy them, as that makes too much debris for viewing). Dump the measured amount of bees into a half-pint or pint jar that will fit to the blender blade assembly. Then add three times as much water as there was bees. Screw on the blade assembly, and hit the “chop” button for two 5-second bursts, making sure that every bee is reduced to tiny pieces.
Grinding a sample of bees in a kitchen blender to release the nosema spores from their guts. One drop of the resulting homogenized slurry is then tested to determine the “average spore count” in millions of spores per bee.
Take the jar off the blender, shake it well, and remove and rinse the blade assembly. If there is much foam in the jar, tip the jar to an angle, and scrape the foam off with a spoon until you see the top of the grayish liquid below. This is the liquid that you view under a scope—all those bees are ground and mixed to yield the one tiny drop of average homogenate that you actually look at!
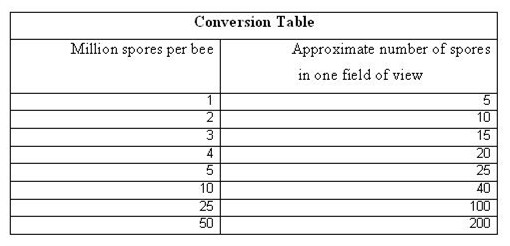
At this point, there are two ways to quantify the infection level—with an expensive hemacytometer glass counting chamber, or on a cheap microscope slide. For management decisions, the slide count is much quicker and easier—you just count the number of spores that you see in one field of view. Thanks to a grant from the California Beekeepers Association, I was able to produce a chart that converts the number of spores in one field of view to approximate millions of spores per bee (realize that even the hemacytometer count is still only an approximation, so don’t get hung up on accuracy, since it is illusory).
Since nosema spores sink quickly, stir the slurry immediately before you take the sample for viewing. Dip the end of a swizzle stick, plastic utensil handle, or the like into the liquid, and place a drop onto a glass slide. Scrape any chunks of bee parts out of the drop, as they will hold up the cover slip too high. Then place a cover slip (I like plastic) over the drop, and tap it down lightly once with your fingertip.
Place the slide under the microscope, but wait at least a full minute for the spores to settle and the liquid to stop moving. Set the scope to 400x (10x top ocular lens, 40x turret objective lens). Look from the side at the space between the lens and the cover slip, and lower the lens until there is just a tiny gap. Then look through the scope, and use the fine focus to bring the slurry into view—you will see pieces of debris and ground bee guts, pollen grains, feathery setae (“hairs”), tracheal tubes, etc. If the view is full of bubbles, prepare a new slide.
Now focus slowly down to the lowest layer of debris—that’s where the nosema spores will sink to. They will be elongated ovals, all roughly the same size. They will always have a dark rim, and generally “glow” white in the center as you move the fine focus up and down. With practice, they will jump out at you!
A standard hemacytometer view of an infection level of about 5 million spores per bee. Each square demarks 1/400th of a square millimeter.
View of a plain microscope slide prep, low nosema infection. Due to camera focus limitations, this is only part of the field of view that you would see if you were to look into the eyepiece. You can see about six spores, a partial pollen grain, and a feathery bee “hair” (seta).
Once you learn to recognize them, you can quickly approximate what your infestation level is by counting how many spores are in the entire round field of view (check a few fields). Don’t get hung up on precise numbers—all nosema counts are only approximations of the actual infection level, no matter what method you use. I received funding from the California State Beekeepers Association to calibrate the “field of view” count vs. the standard hemacytometer counting chamber count. The full paper is on my website, but here’s all you need to know: for every million spores per bee, you’ll see about four or five spores in a field of view.
The big question is, What is level of infestation that I should worry about, or begin treatment? With Nosema apis it was a million (1 x 106) spores. We really don’t know what it should be for N. ceranae. Some authorities suggest the same threshold. I’ve spoken to some large commercial beekeepers, and have tracked nosema counts in my test yard since last fall. It appears to me that colonies can often handle infections levels of 1-5 million spores without much problem.
I’ve followed some thirty infected, but untreated, colonies this season, and watched them build up, often throw infected (5 million spores per bee) swarms, then make good honey crops. As of today’s check in mid September, the only colonies that died went down due to queenlessness or brood diseases. The remainder have spore counts mostly in the 1-6 million range. I’m not sure how these colonies will fare this winter, so I’m going to chicken out and treat most of them, saving five untreated to see what happens.
So I’m not about to make any recommendations. Luckily, in general, the yard samples that I’ve checked for myself or others often have nearly zero spores. In this case, no treatment is indicated. Indeed, there is unpublished data that fumagillin may have a negative effect on colonies, and is not indicated if spore counts are in the low million range. Dr. Frank Eischen presented data last year that indicated that colonies wintering in California built up better with pollen supplement alone, rather than supplement plus fumagillin, provided that spore counts were low.
Treatment
O.K., so let’s say that you have found out which of your colonies (or yards) are actually infected, and that you’re uncomfortable with the spore count. You ask, What treatment(s) will actually work, and how best to apply them? At this time, the only well-documented successful treatment for N. ceranae is fumagillin, sold as Fumagilin-B®. If it is given to infected colonies at normal label rates in gallons of heavy syrup, it is generally effective against ceranae. But not as consistently as it was against N. apis. Also, be aware that since N. ceranae thrives during summer, it can fairly quickly rebound after treatment.
Europeans are more concerned about contamination of the honey with medications, so may feed smaller amounts of syrup weekly, for four weeks. The point is that you must have the medication present through at least one full brood cycle to break the transmission of the infection. Dr. Higes successfully controlled N. ceranae by feeding only 500ml (about 2 cups) of light medicated syrup a week for 4 weeks (at a concentration equivalent to 1 large bottle of Fumagilin-B in 40 gal of syrup).
There are times that feeding gallons of syrup are impractical, such as when colonies are plugged with honey, or during spring or summer when you don’t want to contaminate the honey crop with gallons of medicated syrup. In these cases, many beekeepers are using the “drench” method. In a recent trial, I tested fumagillin as a drench at the colony dosage of 30mg of active ingredient (1 large bottle of Fumagilin-B in 20 gallons of 1:1 syrup, applied at the rate of 1 cup per week for 4 consecutive weeks). This treatment reduced the buildup of the infection, but did not stop it consistently. Apparently, drenching bees does not get the medication to them as effectively as by feeding it in larger quantities of syrup, so the beekeeper must compensate by somewhat increasing the dosage.
A number of commercial beekeepers are having success at drenching with 8-12 oz of medicated syrup, prepared by adding a large bottle of Fumagilin-B to only 5-7 gallons of syrup, and applying two treatments, 10-14 days apart. Note that this method gives about 25% more active ingredient to the colony than by feeding the standard medicated two gallons.

The “drench” method of applying fumagillin—used by some beekeepers when feeding gallons of heavy syrup is impractical. It appears that about 120mg of active ingredient per dose may be necessary by this method of application to control Nosema ceranae, but more research is needed.
I also tested some other treatments. One that showed promise is Nosevit® ( alaskaheavenlyhoney@hotmail.comThis e-mail address is being protected from spam bots, you need JavaScript enabled to view it ), which is widely used in central Europe, but my trial was too small to determine if it was significantly effective. Other treatments, such as thymol, HoneyBHealthy, or bleach have yet to demonstrate efficacy in a controlled trial. There is currently ongoing research worldwide to test other promising nosema treatments.
Bottom Line
There are still a great number unanswered questions about Nosema ceranae. We are not sure exactly what the treatment threshold should be, but it is likely in the 1-5 million spores per bee range. At this level, the vast majority of the bees in the colony may be uninfected—and most of the infection will be in the oldest field bees.
Once N. ceranae is in your operation (if you detect nosema spores during the summer, you can likely assume that it is ceranae), it would be prudent to keep a close eye on it. In order to determine your level of infection (if any), samples should be taken at the entrance at midday, in order to sample the most infected bees. Samples should be taken throughout spring and summer, as well as fall.
As far as treatment, Fumagilin-B is still the proven choice, best administered as per label instructions for the time being. Fumagillin should not be fed unless actual sampling demonstrates that there is indeed an infection, since it may set the colonies back a bit.
When feeding gallons of syrup is impractical, Fumagilin-B can be fed in less syrup or by the drench method, but the concentration of fumagillin needs to be adjusted. As always, consult with your State Apiculturalist for officially recommended treatments. Far more details of every aspect of this article can be found at my website (www.scientificbeekeeping.com), as well as citations for any research mentioned, and current updates.
Further Reading
Higes, M., et al (2008) How natural infection by Nosema ceranae causes
honeybee colony collapse http://www.apitrack.com/pdf/Espania_Enviromental_Microbiology_09_2008.pdf
Matilla, HR and G Otis (2006) The effects of pollen availability during larval development on the behaviour and physiology of spring-reared honey bee
workers. Apidologie 37:533–546.
Pajuelo, AG, C Torres and FJ Orantes Bermejo (2008) Colony losses: a double blind trial on the influence of supplementary protein nutrition and preventative treatment with fumagillin against Nosema ceranae. Journal of Apicultural Research 47(1): 84-86.








